Keywords
Abstract
Interest in van der Waals complexes of oxygen with xenon is due to the alleged participation of such
complexes in providing anesthetic action of xenon in medicine The work is devoted to the measurement of the
intermolecular binding energy in van der Waals complexes of oxygen with xenon in Xen-O2. Van der Waals complexes
of oxygen with xenon were generated in a pulsed molecular beam. The velocity map imaging technique
was used to measure the energy distribution and the angular distribution anisotropy over the recoil directions of
oxygen atoms arising in the photodissociation of these complexes in the Xen-O2+hν→ Xen+O+O process. The angular
distribution over the recoil directions of oxygen atoms with respect to the direction of the polarization of the
exciting radiation indicates the dominant contribution of T-shaped complexes, in which xenon atoms are oriented
perpendicular to the axis of the O2 molecule. At a low xenon content in the expanding gas mixture, the dominant
contribution is provided by T-shaped Xe-O2 complexes with van der Waals binding energy of 156 ± 11 cm‑1.
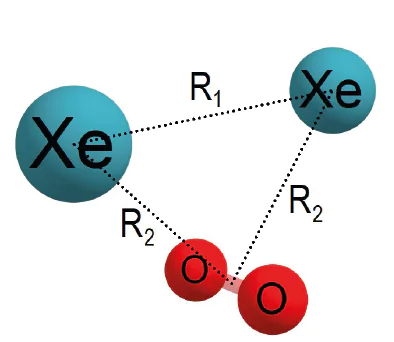
With an increase in the xenon concentration, the T-shaped complexes with higher binding energy appear. It is concluded
that these complexes have structure Xe2-O2. This assignment is confirmed by the measured velocity map
of Xe+ ions which indicates the presence of dimers Xe2 in molecular beam at these conditions. The energy of the
van der Waals binding of O2 with Xe2 in Xe2-O2 complex was determined to be 314 ± 30 cm‑1, and the structure
of these complexes was also proposed.
References