Keywords
Abstract
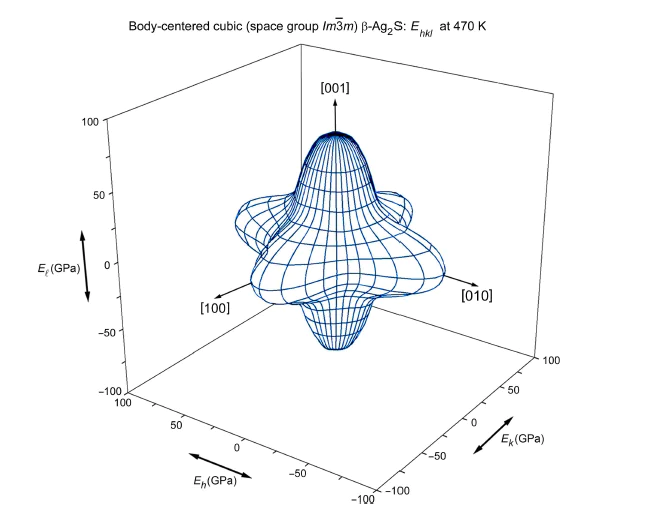
Such phases of silver sulfide as body-centered cubic argentite and monoclinic acanthite are widely known. Traditionally, acanthite is considered as the only low-temperature phase of silver sulfide. Low-temperature monoclinic acanthite can be considered as a result of the ordering of sulfur atoms in a non-metallic volume-centered cubic sublattice of argentite, accompanied by a redistribution of silver atoms. However, the possible existence of other low-temperature phases of silver sulfide cannot be excluded. The search for the model phases of the silver sulfide was performed using an evolutionary algorithm. The possibility of the formation of Ag2S phases with cubic, tetragonal, orthorhombic, trigonal, monoclinic and triclinic symmetries is considered. The calculation of the cohesion energy and enthalpy of formation showed that the formation of low-symmetry phases of Ag2S is energetically most favorable. The elastic stiffness constants cij of all predicted phases of Ag2S are calculated and their mechanical stability is determined. The electron state densities of the predicted Ag2S phases are calculated. Channels of disorder-order transitions associated with the formation of low-temperature unrelaxed monoclinic acanthite ɑ- Ag2S and cubic (space group Pn3m) silver sulfide Ag2S from disordered argentite have been found. The spatial distributions of Young’s modulus and comprehensive compression of cubic (space group Pn3m) silver sulfide Ag2S are determined and a weak anisotropy of its elastic properties is established.